Xibin Zhou and his colleagues in the Kapteyn/Murnane group have come up with a clever new way to study the structure of carbon dioxide (CO2) and other molecules. The researchers use two innovative tools: (1) coherent electrons knocked out of the CO2 molecules by a laser and (2) the X-rays produced by these electrons when they recollide with the same molecules. The coherent electrons and X-rays are produced in a process known as high harmonic generation.
The process involves four steps. First, an intense laser pulse "kicks" the CO2 molecules to set them spinning in such a way that they become aligned with each other. Second, a high-intensity laser pulse plucks the outermost electron out of a CO2 molecule. Third, the laser’s electric field accelerates the free electron. Finally if the free electron comes anywhere near its ionized parent molecule, it crashes back into it and stays there. This high-energy collision causes the reconstituted molecule to emit an X-ray photon.
Zhou and his colleagues showed that the intensity and phase of the emitted X-ray photons depend on the orientation of the CO2 molecules when electrons recollide with them. This means that measurements of the orientation dependence of the intensity of the emitted X-ray beam can provide information about the molecular structure of CO2!
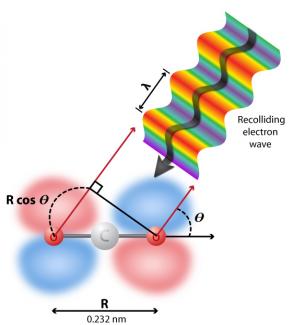
To understand why this is true, it’s useful to consider the graphic on the left, which shows an electron wave function colliding with a CO2 ion (step 4 of high harmonic generation). The grey ball in the center represents a carbon atom, and the two red balls represent oxygen atoms. The red and blue areas are the molecule’s electron clouds, which center on the oxygen atoms. In this figure, a recolliding electron wave function arrives at an obtuse angle to the molecule, making it possible for the crests and troughs of the wave function to interfere with each other inside the CO2 molecule. This interference can either enhance the intensity of the emitted X-rays (constructive interference) or eliminate the X-ray photon emission altogether (destructive interference).
When Zhou and his fellow researchers investigated the electron recollision behavior of CO2 ions with coherent electrons, they not only saw interference fringes, but also observed shifts as small as 5 nm in the location of the fringes when they changed the orientation of the molecules. Their experiment provided the best experimental evidence to date that both the coherent electrons and the intensity and phase of emitted X-rays created during high harmonic generation contain information on the structure of the molecule involved. - Julie Phillips